Product Information
StemFit™ Basic04 Complete Type GMP

StemFit Basic04 Complete Type (Basic04CT) is an animal-origin free, defined medium for human pluripotent stem cell culture. This medium includes Basic Fibroblast Growth Factor (bFGF) can be used for maintaining human ES and iPS cells under feeder-free culture conditions. The QMS (Quality Management System) follows the Ajinomoto System of Quality Assurance (ASQUA). Manufacturing and product release testing of this product are managed in accordance with the GMP standards Ajinomoto formulated for culture media for regenerative medicine. The GMP standards are prepared with reference to ICHQ7 and “PMDA guidance for Aseptic Manufacturing Processes of Regenerative Medicine Products.
StemFit™ Basic04 Complete Type GMP
FAQ
- Are there any changes in media composition? No changes in composition from previous(non-GMP) product.
- What changes were made in production process? Filters used for sterilization of product solutions were conventionally 0.2 µm pore diameter filters. For quality improvement, this has been changed to a filter with a pore size of 0.1 µm, which is capable of capturing mycoplasma. The filter material has not been changed from PES. There are no other changes in the manufacturing process.
- New CoA format? How are QC tests performed?
Test methods for pH, osmolality, sterility, endotoxin, and mycoplasma refer to Japanese Pharmacopoeia (JP). Details of the test methods are given in the new CoA as corresponding JP chapter numbers.
All QC tests are validated. - What is the QMS in place? (Local 9001,ASQUA)?
The QMS (Quality Management System) follows the Ajinomoto System of Quality Assurance (ASQUA). Manufacturing and product release testing of this product are managed in accordance with the GMP standards Ajinomoto formulated for culture media for regenerative medicine.
ASQUA, which means the Quality Assurance system of Ajinomoto Group, consists of common rules in Ajinomoto and actual operating rules in each organization. Common rules consist of Group Shared Policy on quality and safety, Ajinomoto Quality Assurance Regulations, Regulation for Quality Assurance, and ASQUA standards. ASQUA is consistent with ISO9001, as well as manufacturing management standards Hazard Analysis and Critical Control Points (HACCP), food, hygiene management standard, and good manufacturing practices (GMPs)
(For more information: https://www.ajinomoto.com/quality_assurance/ajinomoto-system-of-quality-assurance)
The GMP standards are prepared with reference to ICHQ7 and “Guidance for Aseptic Manufacturing Processes of Regenerative Medicine Products. - How is sterility ensured for regenerative medicine products?
Operator: Training, Qualification
Monitoring: Environment, Operator
Filter: Bacteria challenge test
Process: PST(Process simulation test) - Receiving inspection of raw materials? Appearance test and ID test by Raman spectroscopy or chemical test.
- Can you fill in our supplier questionnaire? Template using Rx-360 Supplier Assessment Questionnaire is available on a request basis. Please contact at stemfit@asv.ajinomoto.com or your usual contact of StemFit™ product.
- Can I audit the manufacturing site? For any audits, please make a prior request of at least 6 months before the desired performance of audits/inspections of the QMS and manufacturing facility.
- I would like to receive media in bags for our production.
Do you have GMP grade bag media? Media in bags are available on a customization basis, please plan ahead for your production schedules, lead times can vary. - Where can I continue to purchase RUO media? or will it be discontinued? We will maintain the supply of both the current and new product until mid-2024.
— Materials Provided —
| Volume | Storage |
| 500 ml | Store at below -20 °C |
— Media Preparation —
StemFit Basic04 Complete Type is provided frozen medium and can be stored at below -20 °C until use. Use sterile techniques when using this medium.
1) Before use, thaw the frozen Basic04CT with occasionally mixing at room temperature (15-25 °C).
CAUTION : Do not thaw, StemFit Basic04 Complete Type at 37 °C, as it accelerates the degradation of the medium.
If precipitations are observed, keep the bottle at room temperature and dissolve them.1
2) Upon thawing, StemFit Basic04 Complete Type may be aseptically aliquoted and stored at below -20 °C. Thawed StemFit Basic04 Complete Type may be stored at 2-8 °C for up to two weeks.2
We recommend storing the medium protected from light.3
3) Before use, warm aliquots at room temperature and use immediately.
Key points for successful culture

Do not thaw, “StemFit Basic04 Complete Type” at 37 °C, as it accelerates the degradation of medium ingredients.

Thawed StemFit Basic04 Complete Type medium may be stored at 2-8 °C for up to two weeks.

We recommend storing the medium protected from light
— Precaution and Disclaimer —
StemFit Basic04 Complete Type is for research use only and is not intended for diagnostic or therapeutic use.
Features
Animal-Origin Free
Minimize risk of virus contamination and lot-to-lot variation with animal-origin free formula.
One Bottle Composition
Achieve sterilization by simplifying the procedure with ready to use packaging.

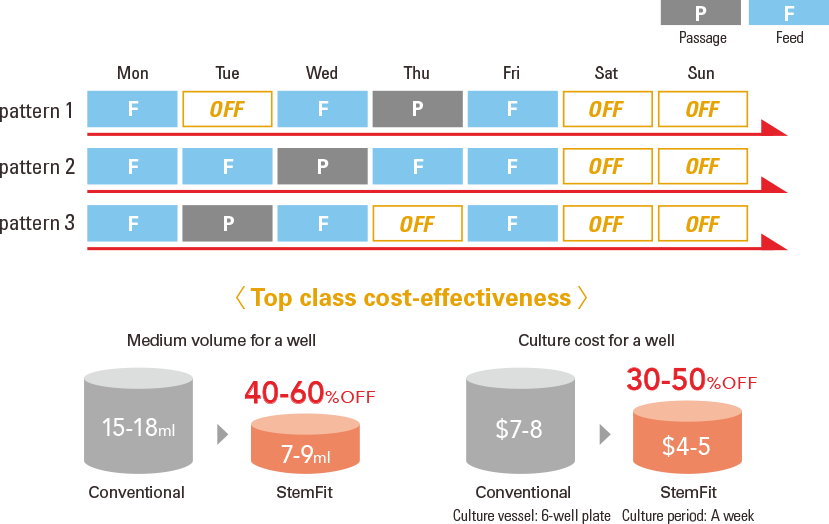
Weekend-Free Feeding
Skip feeding on weekends for flexible scheduling.
Single-Cell Expansion
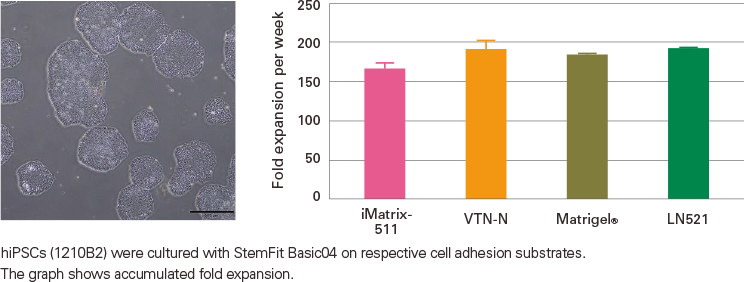
Experience superior expansion with single-cell passaging and consistent performance with various matrices.
- Download
-
・Product Information (PDF [282KB])
・SDS (PDF [389KB] )
・Brochure (PDF [624KB] )
・GMP statement (PDF [623KB] )
・Instruction Manual
StemFit medium (PDF [775KB] )
StemFit medium with iMatrix-511 (PDF [2.4MB] )
For more information, please contact us.
AJINOMOTO CO., INC. AminoScience Division15-1, Kyobashi 1-Chome, Chuo-Ku, Tokyo 104-8315, Japan