The world’s first clinical study of iPSC-derived corneal epithelial cell- An interview with Prof. Ryuhei Hayashi -22th Nov. 2019.

Prof. Ryuhei HayashiGraduate School of Medicine,
Osaka University
In August 2019, Professor Ryuhei Hayashi, Professor Koji Nishida, and co-workers from the Graduate School of Medicine, Osaka University, reported the world’s first transplantation of a corneal epithelial sheet-derived from induced pluripotent stem (iPS) cells. Corneal epithelial sheets from HLA-homozygous iPS cells were produced and transplanted into patients with corneal epithelial stem cell deficiency. The clinical study was approved by the Japanese government in March 2019 and the first transplant was performed in July 2019 with four transplantations planned in total. In the first study, safety was evaluated as the primary endpoint, and efficacy such as improvement of symptoms and visual acuity were evaluated as secondary endpoints.
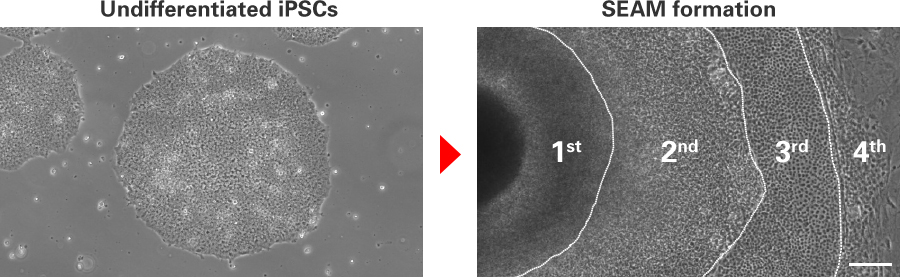
Microscopic images of SEAM (Self-formed Ectodermal Autonomous Multizone) .
Images are kindly provided by Prof. Hayashi
Nature, 531 (7594), 376-80 2016 Mar 17
Overview of clinical study and progress
Currently we are preparing for the second patient. The first operation was successful and as of November 2019, the patient is currently undergoing follow-up, four months after surgery. The second case is scheduled within 2019, and the interim evaluation will be conducted in mid-2020 for the first 2 patients. The first two cases are performed with a protocol that transplants cells-derived from iPS cells whose HLA type is inconsistent with the patient and requires the use of immunosuppressants after surgery. We will analyze the course of the two cases in the interim evaluation to determine whether HLA should be matched and whether immunosuppressants should be used. After that, we plan to perform two additional transplantations in late 2020. The clinical study is planned to be conducted in a total of four cases, and it will be closed in 2021. I think it is important to analyze each case thoroughly, not just the number of operations.
Important and difficult points in clinical cell production
There are so many points. First, stable culture of iPS cells is difficult. In some cases, the growth rate varied depending on the facilities, operators, and locations. The SOP was given as much width as possible, and for instance, the initial number of seeding cells was adjusted between facilities. Instead, we determined the product specification of the iPS cell derived-corneal epithelial sheet as a final product and if the quality was the same between facilities, it was judged as acceptable. It is also important to select an optimal iPS cell line for the project as not all iPS cells can differentiate into the corneal epithelial cells. From the multiple iPS cell lines provided by Kyoto Univ. CiRA, a cell line that stably and efficiently differentiates into the corneal cells was selected. The cell line selected this time showed no genomic mutation (Cosmic census + Shibata list) even in the final product.
Important and difficult points from a legal and regulatory perspective
At the time, there were no established test items required for the application of iPS cells to human patients. (it's not be defined even now). So we shared and exchanged the latest information closely with Dr. Masayo Takahashi (RIKEN), who conducted clinical study on retinal cells derived from autologous iPS cells, and Dr. Jun Takahashi (Kyoto Univ.), who is conducting clinical trial on dopamine neurons-derived from allogeneic iPS cells. In particular, regarding tumorigenicity, the required evaluation method differed depending on the transplantation site, transplantation procedure, and characteristics of the final product. Therefore it is important to design logical evaluation protocols for non-clinical tests and provide data that can prove the safety of cell products. Also, if the cell line is different, non-clinical test data may be required again, even for iPS cells produced by the same production method. So it is necessary to decide the cell line to be used in an actual clinical study as soon as possible. In the framework of clinical study, we consulted with PMDA on Japanese standards for biological materials and referred to their opinions. Ajinomoto StemFit® AK03N (Basic03) and iMatrix-511 had already been approved for clinical use by PMDA, so no additional data or paperwork were required. It was very helpful.
Quality control for iPS cells
iPS cells are discarded at a specific passage number. They are thawed from the frozen cell stock, used as differentiation culture after 1-2 passages, and discarded up to about 10 passages in total. It has been confirmed in advance that corneal epithelium can be obtained stably from iPS cells of up to 10 passages. One cell stock can produce the products for 5-6 patients, including lots for quality control. Regarding scale-up, if the initial number of iPS cells is increased, the amount of the final product is theoretically increased. But in reality, the process of cell sorting for purifying the target corneal epithelial cells becomes a bottleneck. There is a limit to the number of hours of manufacturing time in a day for now.
Selection criteria for culture media and growth factors
When deciding where to buy raw materials, it is a prerequisite that a manufacturer assumes clinical use, and it is important to comply with local regulation such as Japanese standards for biological materials. It is also important that the company cooperate with the authorities for clinical studies or trials. In this project, the transition from pre-clinical to clinical was very smooth since reagents for clinical use were used from the early stage of research. Regarding reagents with lot differences, we want to switch in the future, since enormous effort is required for lot checking. The raw materials used in the early stage of cell production (such as in maintenance culture of iPS cells, and early stage of differentiation etc.) greatly affect the quality of subsequent cell products, so the switching hurdles are very high and careful examination is required.
Reasons for using StemFit medium and feeling of use
Previously, feeder culture was performed, but the StemFit/iMatrix-511 combination showed stable growth. In our case, fortunately, the compatibility with corneal differentiation was very good, it is stable and has no problems. In addition, because Ajinomoto StemFit AK03N (Basic03) has good clinical compatibility, we have been conducting experiments using AK03N (Basic03), a clinical-grade medium, from the basic research stage to eliminate the effects of changing the medium.
Future prospects, issues, etc.
If it is determined that there is no need to match the HLA type of iPS cells with the patient from the results of the current clinical study, we plan to commercialize it with a cell line currently undergoing clinical study. Currently used HLA-homozygous iPS cell line is the most frequent HLA type in Japanese and can cover about 17% of Japanese. At the same time, a project of corneal endothelial regeneration is underway as there are more patients than that of corneal epithelial disease. We would like to submit a clinical protocol for corneal endothelial regeneration within the next 1-2 years since it is expected that the same iPS cell line will be available in corneal epithelial and endothelial regeneration. There is data that can be shared, and we believe that progress will be made efficiently. Research on the differentiation of eye tissues is ongoing. The lens and lacrimal gland are also interesting, and then we are working on the development of new technology.
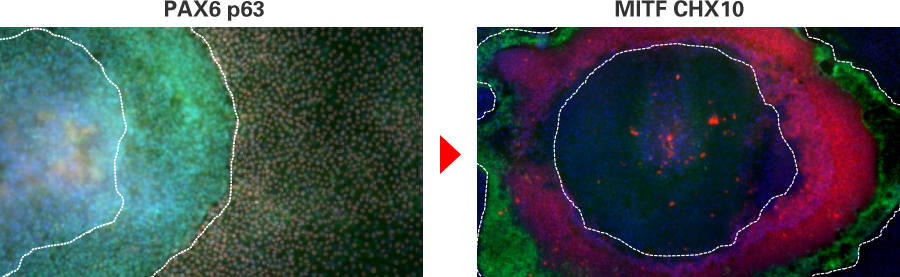
Immunostaining images of SEAM.
Images are kindly provided by Prof. Hayashi
— Related information —
StemFit™ Basic03- Download
-
・Culuture performance of StemFit AK03N(Basic03)(PDF [842kB])
For more information, please contact us.
AJINOMOTO CO., INC. AminoScience Division15-1, Kyobashi 1-Chome, Chuo-Ku, Tokyo 104-8315, Japan



