— Product Size —
10 μg (0.1 mg/ml, 100 μl)
SP-VEGF-R-010UG
50 μg (0.1 mg/ml, 500 μl)
SP-VEGF-R-050UG
1 mg (0.1 mg/ml, 10 ml)
SP-VEGF-R-001MG
GMP compliant product is in preparation. Contact us for further information.
Benefits
Significant cost reduction of cell
manufacturing processes
Seamless transition from basic research
to clinical applications
— Product Details —
| NAME | Recombinant Human VEGF 165 |
|---|---|
| SOURCE | Corynebacterium glutamicum* |
| FORMULATION | Frozen in 20 mM citric acid containing 20 mM GSSG |
| Quality | Animal origin free |
| AA Sequence: | APMAEGGGQN HHEVVKFMDV YQRSYCHPIE TLVDIFQEYP DEIEYIFKPS CVPLMRCGGC CNDEGLECVP TEESNITMQI MRIKPHQGQH IGEMSFLQHN KCECRPKKDR ARQENPCGPC SERRKHLFVQ DPQTCKCSCK NTDSRCKARQ LELNERTCRC DKPRR |
| Purity: | ≥ 95% by SDS-PAGE |
| Endotoxin | <0.1EU/ug |
| Description: | VEGF (Vascular Endothelial Growth Factor) is a protein that plays an important role in angiogenesis, the formation of new blood vessels. It is secreted by cells as a signal to nearby cells to stimulate the growth of new blood vessels. VEGF also plays an important role in other physiological processes such as wound healing, embryonic development, and regulating the permeability of blood vessels. In addition, VEGF has been shown to be involved in the progression of many diseases, such as cancer, diabetic retinopathy, and cardiovascular diseases. Recombinant Human VEGF165 is a 38.2 kDa, disulfide-linked homodimeric protein consisting of two 165 amino acid polypeptide chains. |
*Corynebacterium glutamicum
- • Discovered in 1957 as a glutamate producer
- • Fast growing soil bacterium
- • Gram-positive
- • Non-sporulating
- • Non-pathogenic
- • Non-endotoxic
- • FDA approved

< Features >
- Secretion System
- No Endotoxin
- Less Derivatives (impurities)
- Experience: Food Amino Acid Production
Ideal platform for low-cost, high-grade proteins production with high lot-to-lot consistency.
— Product Comparison Table —
| AJINOMOTO | Company A | Company B | |
|---|---|---|---|
| Host cell | C. glutamicum | E. coli | S. frugiperda |
| Endotoxin | <0.1EU/μg (LAL) |
<1EU/μg (LAL) |
<0.01EU/μg (LAL) |
| Purity | ≧95% (SDS-PAGE) |
≧98% (SDS-PAGE、HPLC) |
>97% (SDS-PAGE) |
| Biological activity |
EC50, 1-30 ng/mL*1 | ED50, 1.0-8.0 ng/ml*2 | ED50, ≦1-6 ng/ml*2 |
| Form | Frozen in 20mM citric acid, 20mM GSSG |
Lyophilized | Lyophilized |
| N-terminal | Ala-Pro-Met | − | Ala |
| Expiration date | 2 years from manufacturing date |
− | 12 months from date of receipt |
- *1 VEGFR2/NFAT reporter assay
- *2 Cell proliferation assay using HUVEC (human umbilical vein endothelial cells)
〈 Comparison of biological activity with other companies’ products 〉
VEGFR2/NFAT reporter assay
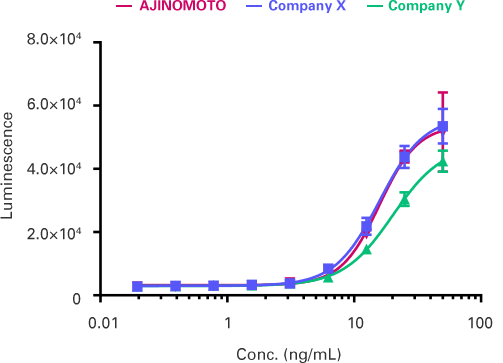
AJINOMOTO’s VEGF demonstrates equal biological
activity compared to tested alternatives!
〈 Stability information after reconstitution 〉
| AJINOMOTO | Company A | Company B | |
|---|---|---|---|
| -80°C | 2 years from manufacturing date |
1 year from reconstitution date |
3 months from reconstitution date (-20 to -70 °C) |
| Freeze-thaw cycles |
Within 3 times* | ー | ー |
As of 2023 Apr.
- * We do not guarantee the product quality.
- Download
-
・Product Information
VEGF [1mg](PDF [260KB]) VEGF [10μg](PDF [270KB]) VEGF [50μg](PDF [272KB])・SDS (PDF [130KB] / En) (PDF [142KB] / JP)
For more information, please contact us.
AJINOMOTO CO., INC. AminoScience Division15-1, Kyobashi 1-Chome, Chuo-Ku, Tokyo 104-8315, Japan