— Product Size —
Activin A
10 μg (0.1 mg/ml, 100 μl)
SP-ActA-R-010UG
50 μg (0.1 mg/ml, 500 μl)
SP-ActA-R-050UG
1 mg (0.1 mg/ml, 10 ml)
SP-ActA-R-001MG
GMP compliant Activin A
1 mg (0.1 mg/ml, 10 ml)
SP-ActA-G-001MG
Benefits
Significant cost reduction of cell
manufacturing processes
Seamless transition from basic research
to clinical applications
— Product Details —
| NAME | Recombinant human Activin A |
|---|---|
| SOURCE | E. coli |
| FORMULATION | Frozen in 50 mM sodium acetate buffer, pH4.6 |
| Quality | Animal origin free |
| AA Sequence: | GLECDGKVNI CCKKQFFVSF KDIGWNDWII APSGYHANYC EGECPSHIAG TSGSSLSFHS TVINHYRMRG HSPFANLKSC CVPTKLRPMS MLYYDDGQNI IKKDIQNMIV EECGCS |
| Purity: | ≥ 97% by SDS-PAGE |
| Endotoxin | <0.01EU/ug |
| Description: | Activin A is a member of the TGF-beta superfamily of cytokines and is involved in a wide range of biological processes including tissue morphogenesis and repair, fibrosis, inflammation, neural development, hematopoiesis, reproductive system function, and carcinogenesis. Human Activin A is a 26.0 kDa disulfide-linked homodimer of two βA chains, each containing 116 amino acid residues. Activin A is mainly used for stem cell cultivation in order to differentiate the stem cell into endoderm or mesoderm. |
— Product Comparison Table —
| AJINOMOTO | Company A | Company B | Company C | |
|---|---|---|---|---|
| Host cell | E. coli | E. coli | CHO | Human |
| Endotoxin | <0.01EU/μg (LAL) |
<1EU/μg (LAL) |
<0.01EU/μg (LAL) |
<0.01EU/μg (LAL) |
| Purity | ≧97% (SDS-PAGE) |
≧97% (SDS-PAGE、HPLC) |
>95% (SDS-PAGE) |
>95% (SDS-PAGE) |
| Biological activity*1 |
ED50, 50-150% vs. WHO std*2 (K562) |
ED50, ≦2.0 ng/ml (MPC-11) |
ED50, 0.2-1.2 ng/ml (K562) |
ED50, 0.5-5 ng/ml (MPC-11) |
| Form | Frozen (0.1 mg/ml) |
Lyophilized | Lyophilized | Lyophilized |
| N-terminal | Gly-Leu-Glu | ー | Gly | ー |
| Expiration date | 4 years from manufacturing date |
ー | 1 year from date of receipt |
1 year from date of receipt |
As of 2023 Apr.
- *1 Measured by its ability to induce hemoglobin expression in K562 human chronic myelogenous leukemia cell.
- *2 Activin A WHO international std., NIBSC code: 91/626
| AJINOMOTO | Company A | Company B | Company D | |
|---|---|---|---|---|
| Host cell | E. coli | E. coli | CHO | E. coli |
| Endotoxin | <0.01EU/μg (LAL) |
≦0.10EU/μg (LAL) |
<0.10EU/μg (LAL) |
<0.05EU/μg (LAL) |
| Purity | ≧97% (SDS-PAGE) |
≧98% (SDS-PAGE、HPLC) |
>97% (SDS-PAGE) |
≧97% (SDS-PAGE) *include ≦5% oligomers |
| Biological activity*1 |
ED50, 50-150% vs. WHO std*2 (K562) |
ED50, 1.0-5.0 ng/ml (MPC-11) |
ED50, 0.2-1.2 ng/ml (K562) |
0.6–2.5x103 IU/mg (MPC-11) |
| Mycoplasma | Negative (NAT) |
Negative (QF-PCR) |
Negative (Ribosomal RNA hybridization assay) |
ー |
| Sterility | Conform (Membrane filter) |
No growth (Direct inoculation) |
ー | No growth (Direct inoculation) |
| Form | Frozen (0.1 mg/ml) |
Lyophilized | Lyophilized | Lyophilized |
| N-terminal | Gly-Leu-Glu | Gly-Leu-Glu | Gly-Leu-Glu | Met-Gly-Leu-Glu |
| Expiration date | 4 years from manufacturing date |
5 years from manufacturing date |
3 years from bottling date |
Minimum 6 months from shipping date |
As of 2023 Apr.
- *1 Measured by its ability to induce hemoglobin expression in K562 human chronic myelogenous leukemia cell.
- *2 Activin A WHO international std., NIBSC code: 91/626
〈 Comparison of biological activity with other companies’ GMP products 〉
Measured by its ability to induce hemoglobin expression in K562 human chronic myelogenous leukemia cell.
Activin A WHO international std., NIBSC code: 91/626
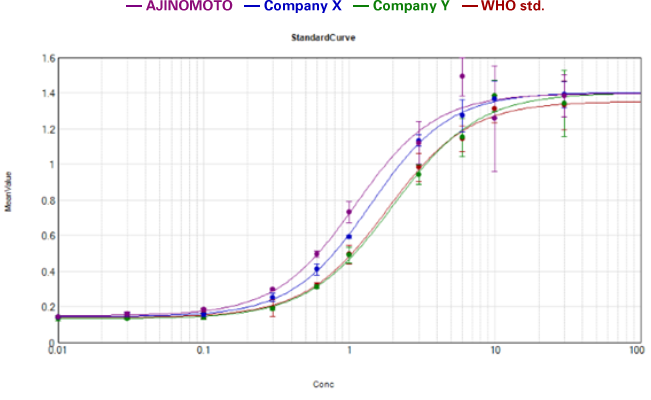
AJINOMOTO’s Activin A demonstrates equal to
superior biological activity compared to tested alternatives!
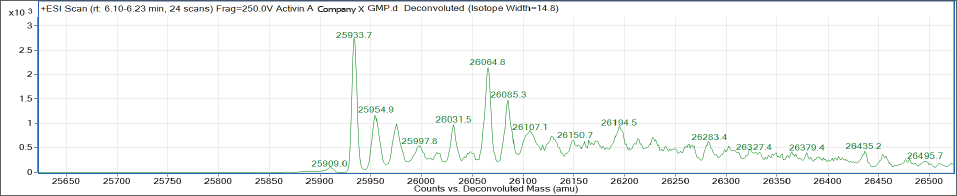
〈 Comparison of MS spectrum with other companies’ GMP products 〉
AJINOMOTO
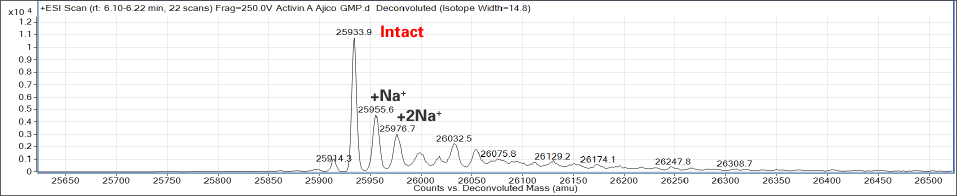
Company X
Company Y
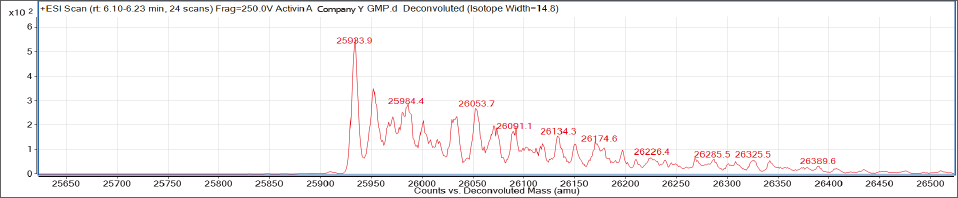
AJINOMOTO’s Activin A also contains significantly less impurities!
〈 Stability information after reconstitution 〉
| AJINOMOTO | Company A | Company B | |
|---|---|---|---|
| -80°C | 4 years from manufacturing date |
At least 7 months | 3 months |
| 4°C | At least 6 months* | 1 week | 1 month |
| Freeze-thaw cycles | Within 2 times* (>90% activity) |
ー | ー |
As of 2021 Oct.
- * We do not guarantee the product quality.
— Clinical Compatibility —
The PMDA* has officially confirmed the eligibility of Ajinomoto’s Activin A for use in clinical cell therapy production (in Japan).
* Pharmaceuticals and Medical Devices Agency

- Download
-
・Product Information Activin A [1mg GMP compliant](PDF [286KB]) Activin A [1mg](PDF [281KB]) Activin A [10μg](PDF [286KB]) Activin A [50μg](PDF [284KB])
・SDS (PDF [74KB] / En) (PDF [123KB] / JP)
For more information, please contact us.
AJINOMOTO CO., INC. AminoScience Division15-1, Kyobashi 1-Chome, Chuo-Ku, Tokyo 104-8315, Japan